High Pressure Single Molecule FRET
Spectroscopic investigations at high pressure help to get a better understanding of biological molecules. For example to get a better understanding of protein folding, the protein has to be unfolded first. This can be done by temperature, the addition of denaturants, pH-changes or pressure. In contrast to other unfolding methods, pressure unfolding is clean, reversible and affects internal interactions exclusively by the changes in the distances of the components, whereas the total energy remains almost constant .
Single molecule FRET (smFRET) has proven a powerful method for the study of the unfolded state of proteins. The unfolded or denatured state of a protein is not a single fixed state, but a collection of states of very similar energies that are in very rapid equilibrium with each other. The denatured states are of special interest, because they are the starting point of the protein folding reaction and the stability is defined as the difference in free energies between the native and the denatured states. In cells the native and the denatured state are in dynamic equilibrium. There are also some proteins that can only cross a lipid bilayer after reconverting into a partially folded or denatured conformation. Additionally, the denatured state is the primary target for degradative enzymes.
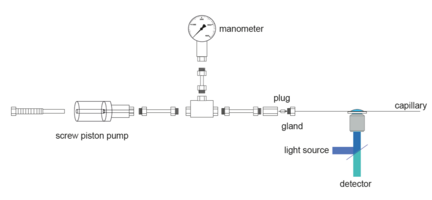
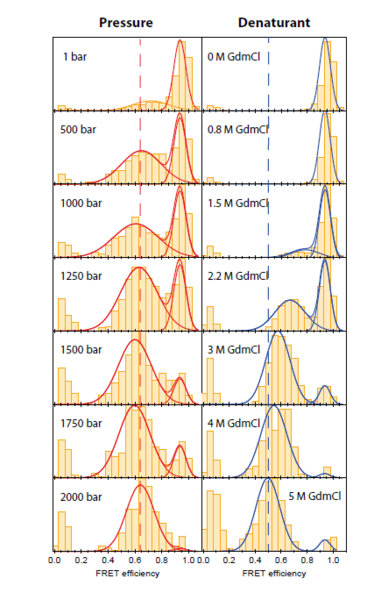
Unfolding by temperature and chemical denaturants has been elucidated by smFRET. Unfolding by high pressure, however, has not been demonstrated to date. We developed a method for smFRET measurements under high pressures (up to 4 kbar) based on a silica capillary as a sample container. The dimensions of the capillary with a wall thickness comparable to the thickness of standard microscope coverslips allow for their use on microscopes with high NA water immersion microscope objectives.
Kim Colin Reiter

Gebäude 61
,
Raum 218
reiter(at)physik.uni-luebeck.de
+49 451 3101 4211

